Welcome to Sciemce, the ultimate destination for finding answers to all your questions.
Determine convergence or divergence of the alternating series.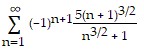
A. Converges B. Diverges
Compared to those of the Middle Stone Age, Late Stone Age and Upper Paleolithic tools __________
a. were crafted with less skill b. were less standardized c. changed more rapidly and more frequently d. included less tools made from shell and bone
A gift to charity from its 2020 income is deductible on an estate’s Form 1041 if it is made by the end of the ____________________ (2020, 2021) tax year.
Fill in the blank(s) with the appropriate word(s).
The Elliptical Marquee Tool allows you to select a(n) ____ area.
a. elliptical b. polygonal c. oval d. circular
As women's roles have become broader and more encompassing, their lives have become ________
A) freer B) overburdened C) more powerful D) happier
Defense mechanisms are considered to be maladaptive when they:
a. are evoked in nonstressful situations b. are unconscious decisions c. become the only way of responding to a threat d. do not protect the mind from stressors
According to your text, all of the following were admitted as refugees during the 1950s except
A) Vietnamese. B) Hungarians. C) Cubans. D) Rwandans.
Unemployment causes a crisis-like situation in nearly all families
Indicate whether the statement is true or false
, A definition of a variable outside any function is called a
a) local function definition b) global variable definition c) global function header d) global function definition e) local variable definition
Outcome research is that which
a. compares outcomes between different populations. b. evaluates the effectiveness of a treatment. c. determines the most efficient target behaviors. d. analyzes how the therapy works.
Which of the following is an example of a persecutory delusion?
a. Believing you are the Savior of the World b. Believing that the government is tapping your phones c. Believing that a movie star is in love with you d. All of these are persecutory delusions
A client had a dislocated shoulder, and when healing, the client had insufficient deposits of collagen during the repair stage. What complication is the nurse aware can occur from this lack of collagen?
A) Carpal tunnel syndrome B) Compartment syndrome C) Volkmann's contracture D) Recurrent dislocations