You wish to drop an ice cube into a cup of hot water to cool the water. The temperature of the ice cube is -10°C, and the water temperature is 92°C. Convert both of these temperatures to Kelvin, and determine the difference between the temperatures in both K and °C.
Given: Tice (°C) = -10°C; Tw (oC) = 92°C
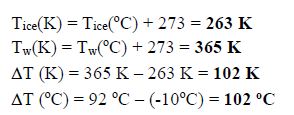
Trades & Technology
You might also like to view...
What does “let the land shape your plan” mean?
What will be an ideal response?
Trades & Technology
PAC uses a very concentrated column of high-velocity, high-temperature _____ gas to rapidly melt or vaporize the metal to create a cut.?
A. ionized? B. plasma? C. molten? D. concentrated?
Trades & Technology
A(n) ____________________ is an ignition system in which the spark is created by an electric current
generated by electrical induction.
Fill in the blank(s) with the appropriate word(s).
Trades & Technology
How do (a) draft and (b) cold floor surfaces cause discomfort for a room’s occupants?
What will be an ideal response?
Trades & Technology