Determine the PBR for the oxidation of nickel to form NiO that has a density of 7.45 g/cm3 .
What will be an ideal response?
Work this problem on a molar basis. One mole of Ni after oxidation results in one mole of NiO. The volume of one mole of Ni is found from the mass divided by the density. The mass of one mole of Ni is equal to the molar mass.
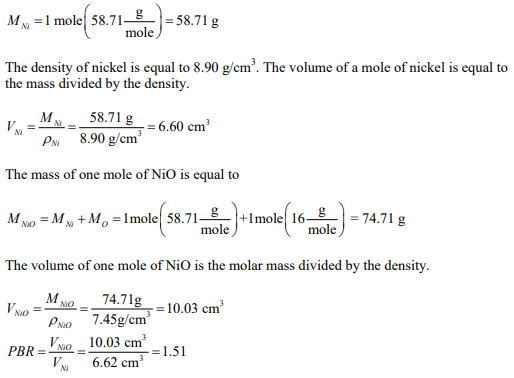
You might also like to view...
An LR circuit is prepared for an experiment, initially with its switch open. The time constant is that time after switch closure at which the energy stored in the inductor reaches what percentage of its final value
A. 63% B. 14% C. 37% D. 40% E. 100%
In epoxy and polyester thermoset polymers, liquid resin and liquid _________are mixed to form a liquid that transforms to a solid.
Fill in the blank(s) with the appropriate word(s).
Center of Mass: Three small masses are positioned at the following coordinates: 3.0 kg at (3.0 m, 2.0 m); 4.0 kg at (0.0 m, -1.0 m); and 5.0 kg at (5.0 m, -7.0 m). What are the coordinates of the center of mass (or center of gravity) of this system?
Fill in the blank(s) with the appropriate word(s).
Spherical particles of density 2.0 g/cm3 are shaken in a container of water (viscosity = 1.0 × 10^-3 N•s/m3). The water is 8.0 cm deep and is allowed to stand for 15 minutes. What is the greatest terminal velocity of the particles still in suspension at that time?
a. 0.55 × 10^-5 m/s c. 4.4 × 10^-5 m/s b. 1.1 × 10^-5 m/s d. 8.8 × 10^-5 m/s