The Pauli Exclusion Principle states
a. no two electrons in the same atom can have the same set of quantum numbers.
b. there is an inherent uncertainty in the position and momentum of a particle.
c. when an atom has orbitals of equal energy, the maximum number of electrons will have unpaired spins.
d. when an atom has orbitals of equal energy, the maximum number of electrons will be paired spins.
e. no two atoms can have the same set of quantum numbers.
a
You might also like to view...
A transverse periodic wave is represented by the equation y(z, t) = 2.0 cm sin(1,200 rad/s t - 20.0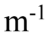 z). Another transverse wave is represented by the equation y(z, t) = 2.0cm sin(1,200 rad/s t + 20.0
z). Another transverse wave is represented by the equation y(z, t) = 2.0cm sin(1,200 rad/s t + 20.0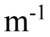 z). What is the equation that represents the superposition of the two waves?
z). What is the equation that represents the superposition of the two waves?
A. y(z, t) = 4.0 cm sin(1,200 rad/s t - 20.0 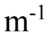 z)
z)
B. y(x, t) = 4.0 cm sin(1,200 rad/s t) cos(20.0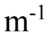 x)
x)
C. y(x, t) = +4.0 cm cos(1,200 rad/s) sin(20.0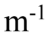 x)
x)
D. y(x, t) = +4.0 cm cos(1,200 rad/s) + (20.0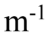 x)
x)
A cold region of dust which scatters the light of nearby stars produces a nebula which appears ________ in color, such as the one encompassing the Pleiades cluster
Fill in the blank(s) with correct word
An object attached to a spring is pulled across a horizontal frictionless surface. If the force constant (spring constant) of the spring is 45 N/m and the spring is stretched by 0.88 m when the object is accelerating at
what is the mass of the object? A) 28 kg B) 24 kg C) 31 kg D) 36 kg
A sound wave in air travels at a speed of 340 m/s. If the wavelength is 0.100 m, in what range of the sound spectrum is the wave?