In the above model of an atom, if you were to draw an electron’s transition from the second excited state to the third excited state, what numbers would this correspond from/to? What kind of spectrum would be produced?
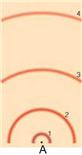
a. 3 to 4, absorption
b. 2 to 3, absorption
c. 3 to 4, emission
d. 2 to 3, emission
A
You might also like to view...
Which of the following is considered by biologists to be a likely place where life might have first arisen on Earth?
A) in hot water near undersea volcanoes B) on meteorites that landed on Earth C) on land surfaces that got moderately heavy rainfall D) deep in Earth's core
Charles Messier mapped the night sky and identified many objects now known to be emission nebulae in his search for objects that might be confused with:
A) comets. B) asteroids. C) galaxies. D) dark nebulae. E) reflection nebulae.
To reach the nearest known exoplanet using current technology would take:
A) a matter of months. B) 4.4 years. C) about a century. D) about 40,000 years. E) close to a million years.
Star #1 is approaching the Earth with speed v. Star #2 is receding from the Earth with the same speed v. Measurements of the same spectral line from each of the stars show Doppler shifts in frequency. The light from which star will have the larger magnitude shift in frequency?
a. star #1 b. Both stars will have the same shift. c. The value of the speed must be known before an answer can be found. d. star #2