Assuming the radius of diatomic molecules is approximately 1.0 × 10-10 m, for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m?
The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ? atm/mol ? K.
A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm
A
You might also like to view...
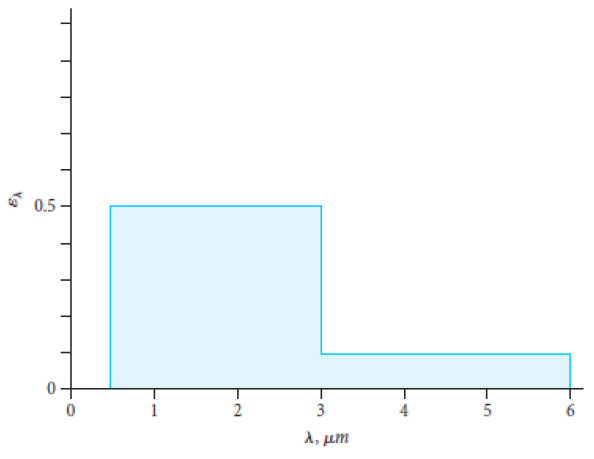
For the gray opaque surface having the spectral emissivity given in the figure, determine the spectral reflectivity.
You drop a freely falling body at the equator and at the north pole. The acceleration of the freely falling body at the equator is _______________ the acceleration of the freely falling body at the north pole
Fill in the blank(s) with correct word
10?6 phones is equal to _______.
A. Mphone B. kphone C. mphone D. ?phone
Which mission has recently provided scientists with information about Mercury's magnetic field?
a. Magellan b. MESSENGER c. LCROSS d. Voyager e. Apollo