A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is how much?
?
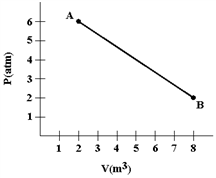
?
a.
?2.42 × 106
b.
?1.40 × 106
c.
?1.02 × 106
d.
1.02 × 106
e.
1.40 × 106
b
You might also like to view...
An object weighing 4.00 N falls from rest subject to a frictional drag force given by Fdrag = bv2, where v is the speed of the object and What terminal speed will this object approach?
A) 1.78 m/s B) 3.42 m/s C) 1.15 m/s D) 2.25 m/s E) 0.75 m/s
The rotation curve of a galaxy is displayed as a plot of
a) gravity vs. distance to galaxy center b) rotation velocity vs. distance from Earth c) period of rotation vs. distance from Earth d) redshift vs. blueshift e) rotation velocity vs. distance to galaxy center
How many moles of air must escape from a 10 m × 8.0 m × 5.0 m room when the temperature is raised from 0°C to 10°C? Assume the pressure remains unchanged at one atmosphere while the room is heated
a. 1.3 × 10^3 moles c. 6.5 × 10^2 moles b. 1.2 × 10^3 moles d. 3.7 × 10^2 moles
What aspect of Kepler's theory would have horrified all astronomers before Kepler?
A) Kepler's elliptical orbits. B) The fact that the Earth has a spherical shape in Kepler's theory. C) Kepler's simple circular orbits. D) The fact that Earth was no longer at the center. E) The absence of any epicycles [or "circles-within-circles"].