In a thermodynamic process involving 7.8 moles of an ideal gas, the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3
The gas expands adiabatically to a volume of 0.080 m3. For this gas, CV = 12.27 J/mol • K, and the ideal gas constant is R = 8.314 J/mol ? K. Calculate the work done by the gas during this expansion.
11 kJ
You might also like to view...
Heat Engines: The figure shows a pV diagram for a cycle of a heat engine for which QH = 59 J. What is the thermal efficiency of the engine?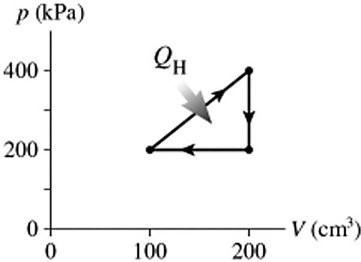
A. 17% B. 34% C. 8.5% D. 14%
?
A. animated B. scripted C. dynamically generated D. static
Element A, mass 9.00 g, reacts exactly with 5.00 g of element B to form 14.00 g of compound AB. If 36.00 g of element A is reacted with 41.00 g of element B to form the same compound, what mass of compound AB will be formed?
a. 9.00 g b. 56.00 g c. 9.00 g d. 30.00 g
You observe a rocket moving away from you. Compared to its length when it was at rest on the ground, you will measure its length to be
1.shorter. 2.longer. 3.the same.