A monatomic ideal gas is compressed adiabatically to one-third of its initial volume. The resulting pressure will be
A) three times as large as the initial value.
B) less than three times as large as the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value.
E) impossible to predict on the basis of this data.
C
You might also like to view...
The specific heat of ice is 2.10 kJ/kg  the heat of fusion for ice at 0
the heat of fusion for ice at 0 is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg
is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg  and the heat of vaporization of water at 100
and the heat of vaporization of water at 100 is 2,256 kJ/ kg. What is the final equilibrium temperature when 30.0 grams of ice at -15.0
is 2,256 kJ/ kg. What is the final equilibrium temperature when 30.0 grams of ice at -15.0 is mixed with 8.00 grams of steam at 100
is mixed with 8.00 grams of steam at 100 ?
?
A. 65.6
B. 60.2
C. 56.2
D. 50.1
E. 45.2
The possibility of life being transferred from another planet like Mars largely depends on whether that life can survive
A) being lasted from the parent planet B) inside the meteorite with very little water present C) long enough in space to make the journey to Earth D) the impact onto the Earth's surface
What is the potential difference VB ? VA when I = 1.5 A in the circuit segment below?
?
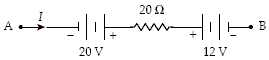
?
a.
+22 V
b.
?22 V
c.
?38 V
d.
+38 V
e.
+2.0 V
According to a postulate of Einstein, which of the following describes the nature of the laws of physics as one observes processes taking place in various inertial frames of reference?
a. Laws are the same only in inertial frames moving at near speed of light. b. Laws are the same in all inertial frames. c. Laws are the same only in inertial frames with zero velocity. d. Laws are the same only in inertial frames moving at low velocities.