What state (compressed liquid, sat liquid, sat vapor, mix of sat liquid and sat vapor with a certain quality or super heated vapor) is the benzene in at this T and P? How do you know? Please explain/provide convincing evidence.
You are planning a DEMO experiment and would like to estimate the pressure exerted by 4.5 g grams of benzene at 150 °C in a rigid 1 liter container using two approaches:
(a) Use the ideal gas law.
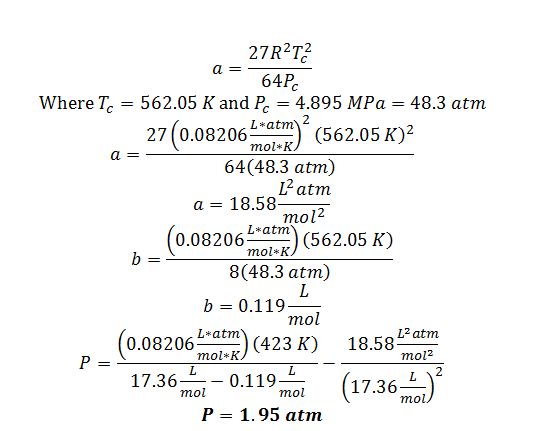
(b) Use the van der Waals equation of state where a=18.24 (L^2*atm)/?mol?^2 and b=0.1154L/mol for benzene.
Apply ideal gas law:
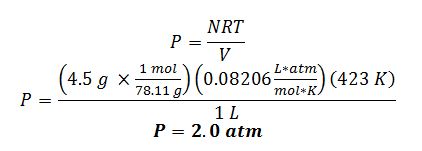
Apply van der Waals equation of state:
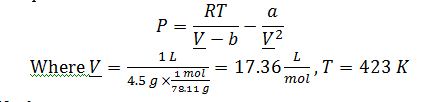
Solve for Van Der Waals parameters:
Solve for vapor pressure of benzene @ 150 °C using the shortcut equation:
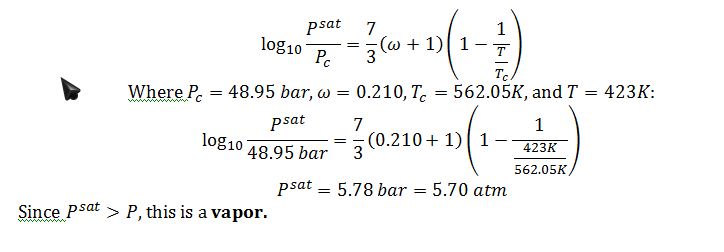
You might also like to view...
Describe the Design-Build contracting method.
What will be an ideal response?
Feed additives are used in animal rations because they promote faster gains, improve feed efficiency,
and/or _____.
a. improve hormone production c. improve animal health b. lower feed costs d. improve fertility
A tire is inflated to a pressure of 35.0 psi. How much weight does the tire support it contacts the ground with an area of 30.0 in^2?
What will be an ideal response?
Technician A says a fully engaged clutch friction disc must slip slightly to absorb engine vibrations Technician B says the clutch friction disc is designed to slip slightly during engagement and disengagement to provide smooth operation. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians A and B D) Neither technician A nor B