One mole of air, initially at 150°C and 8 bar, undergoes the following mechanically reversible changes. It expands isothermally to a pressure such that when it is cooled at constant volume to 50°C its final pressure is 3 bar. Assuming air is an ideal gas for which 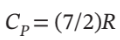 and
and 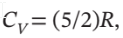 calculate W, Q, ?U, and ?H.
calculate W, Q, ?U, and ?H.
What will be an ideal response?
For the initial state of 150 °C and 8 bar, the molar volume is
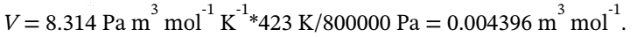
If the final state is 50 °C and 3 bar, then the molar volume at the final state is
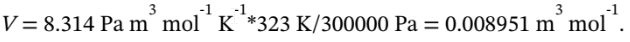
This is also the molar volume at the intermediate state, since the gas goes from the intermediate state to the final state at constant volume. The temperature at the intermediate state is 150 °C, since the gas goes from the initial state to the
intermediate state isothermally. So, the pressure at the intermediate state is
P = 8.314 Pa
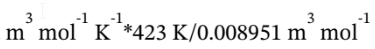 = 392897 Pa = 3.929 bar.
= 392897 Pa = 3.929 bar.
For the isothermal expansion, ?U = ?H = 0 and Q =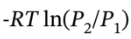 = -R*423 K *ln(3.929 bar/8 bar) = 2501 J/mol, and
= -R*423 K *ln(3.929 bar/8 bar) = 2501 J/mol, and
W = -Q = -2501 J/mol.
For the isochoric cooling, ?U = 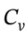 ?T = 2.5 R*(323 K-423 K) = -2079 J/mol, and
?T = 2.5 R*(323 K-423 K) = -2079 J/mol, and
?H = 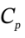 ?T = 3.5 R*(323 K – 423 K) = -2910 J/mol. At constant volume, W = 0, so Q = ?U = -2079 J/mol.
?T = 3.5 R*(323 K – 423 K) = -2910 J/mol. At constant volume, W = 0, so Q = ?U = -2079 J/mol.
For the overall 2-step process, we then have
?U = -2079 J/mol, ?H = -2910 J/mol, Q = 422 J/mol, and W = -2501 J/mol.
You might also like to view...
An ancient Egyptian papyrus provides evidence of ____ for royalty, as Ankhesenamom brings
papyruses and lotto flowers to Tutankhamen.
a. floral offerings c. mating rituals b. temple decorations d. cultural education
Until about 6 months old, a horse is called a(n) ____________________ or ____________________.
Fill in the blank(s) with the appropriate word(s).
If a person came into contact with a 120 volt potential, the current flow ______________.
A. wouldn't be noticeable B. will hardly be felt C. will hurt but not cause any damage D. has the potential to be fatal
How are environmental concerns changing the role of construction professionals?
What will be an ideal response?