What is the change in entropy of the lead when 2.0 kg of molten lead solidifies? [Data: Lv = 207. kcal/kg at 1744.°C; Lf = 5.9 kcal/kg at 328.°C]
What will be an ideal response?
-0.020 kcal/°K
You might also like to view...
The Clausius form of the second law of thermodynamics states that
A. it is impossible to make heat flow from a colder to a hotter body B. heat naturally flows from a colder to a hotter body C. heat will not flow from a colder to a hotter body unless some other effect is involved
Liquid sodium is to be heated from 500 K to 600 K by passing it at a flow rate of 5.0 kg/s through a 5-cm-ID tube whose surface is maintained at 620 K. What length of tube is required?
GIVEN

FIND
The length of tube (L) required
ASSUMPTIONS
Surface temperature is constant and uniform
SKETCH
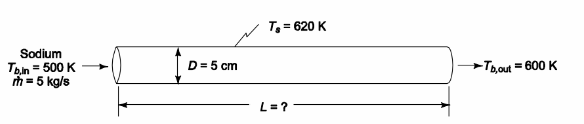
PROPERTIES AND CONSTANTS
for liquid sodium at the average bulk temperature of 550 K
Arsenic-doped silicon is
A) a material with holes as the dominant charge carrier. B) an n-type semiconductor. C) a p-type semiconductor. D) all of the above. E) none of the above.
A series circuit consists of a 12.0 V source of emf, a 2.00 mF capacitor, a 1000  resistor, and a switch. What is the time constant for this circuit?
resistor, and a switch. What is the time constant for this circuit?
A. 10.0 s B. 2.00 s C. 0.693 s D. 0.0825 s E. 1.00 ms