Solve the problem.The hydrogen ion concentration of a substance is about 2.9 x 10-12 moles per liter. Find the pH. Round to the nearest tenth. Use the formula pH = -log [H+].
is about 2.9 x 10-12 moles per liter. Find the pH. Round to the nearest tenth. Use the formula pH = -log [H+].
A. -12.5
B. 12.5
C. 11.5
D. 11.8
Answer: C
You might also like to view...
State the degree and leading coefficient of the polynomial function.f(x) = 16x4 - 5x + 5
A. Degree: 5; leading coefficient: 16 B. Degree: 6; leading coefficient: 16 C. Degree: 4; leading coefficient: 16 D. Degree: 16; leading coefficient: 4
Decide whether or not the function is continuous in the indicated x-interval.-4 to -3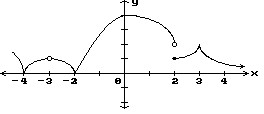
A. Not continuous B. Continuous
The exterior sides of adjacent angles 1 and 2 are perpendicular. If 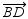

src="https://sciemce.com/media/4/ppg__cognero__Section_1.4_Relationships_Perpendicular_Lines__media__49e5a047-ac25-43c0-b80d-ca692bfeab0f.PNG" />. What will be an ideal response?
Change the percent to a fraction or a mixed number in lowest terms.29%
What will be an ideal response?