Helium gas has the special property that its thermal energy is directly proportional to its Kelvin temperature. Consider a flask of helium with a temperature of 10°C
If it is heated until it has twice as much thermal energy, show that its temperature is 293°C. Why would doubling the thermal energy of a flask of 10°C water not produce the same temperature?
What will be an ideal response?
Answer: Thermal energy changes are directly proportional to absolute temperature changes. So 10°C is 283 kelvins, and two times 283 kelvins = 566 kelvin. So helium with doubled thermal energy would have a temperature of 566 kelvin. Converting this to Celsius means subtracting 273 from 566, which gives 293°C. For water there is the added complication of phase change. Phase change for water is so great that doubling the thermal energy of a flask of 10°C water would be part water and part steam at 100°C!
You might also like to view...
A single slit, which is 0.0500 mm wide, is illuminated by light of 550. nm wavelength. What is the angular separation between the first two minima on either side of the central maximum?
What will be an ideal response?
Determine the energy stored in C2 when C1 = 15 ?F, C2 = 10 ?F, C3 = 20 ?F, and V0 = 18 V
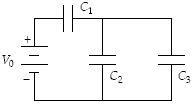
a.
0.72 mJ
b.
0.32 mJ
c.
0.50 mJ
d.
0.18 mJ
e.
1.60 mJ
What keeps the moon moving, i.e., why doesn't the moon slow down and stop?
A) It actually is slowing down, and will come to rest in a few thousand more years. B) Nuclear processes operating in the central core of the moon. C) It is the natural motion of every object to keep moving by itself. D) The sun's gravity keeps it moving. E) Earth's gravity keeps it moving.
As you go from a point where a gravitational field is strong to a point where the gravitational field gets weaker, the gravitational field lines get farther apart
a. True b. False Indicate whether the statement is true or false