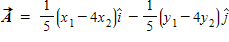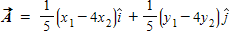Energy Levels: In the n = 1 state, the energy of the hydrogen atom is -13.6 eV. What is its energy in the n = 2 state?
A. -6.79 eV
B. -4.53 eV
C. -3.40 eV
D. -1.51 eV
Answer: C
You might also like to view...
Exoplanets known as super-Earths
A) orbit extremely massive stars. B) have masses comparable to that of Jupiter. C) have masses 2-10 times that of Earth. D) have masses comparable to that of Neptune. E) have yet to be observed.
A container with rigid walls is filled with 4.00 mol of air at 17°C with CV = 2.5R. What is the final temperature of the air if its internal energy is increased by 28 kJ? (R = 8.31 J/mol ? K)
A) 337°C B) 354°C C) 337 K D) 354 K E) 610 K
Why might we think that silicon would be an obvious alternative to carbon as a building block for biological molecules?
A) it forms the basis of some life forms on Earth B) it has a similar abundance on Earth to carbon C) it forms complex molecules like carbon D) it has a similar electronic structure to carbon, forming a maximum of four bonds and, hence, should have a similar chemistry
Instructions: On occasion, the notation 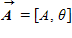
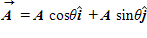
Given that 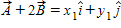
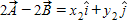
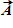
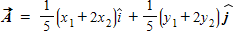
B.
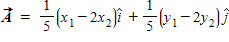
C.
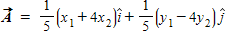
D.
E.