An ideal gas is to taken reversibly from state i, at temperature T1, to any of the other states labeled I, II, III, IV, and V on the p-V diagram below. All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest.
A. I, II, III, IV, V
B. V, IV, III, II, I
C. I, then II, III, IV, and V tied
D. I, II, III, and IV tied, then V
E. I and V tied, then II, III, IV
Answer: A. I, II, III, IV, V
You might also like to view...
Charge in an Electric Field: An electron is initially moving to the right when it enters a uniform electric field directed upwards, as shown in the figure. Which trajectory (X, Y, Z, or W) will the electron follow in the field?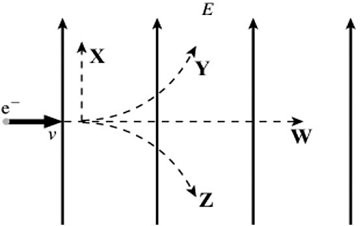
A. trajectory W B. trajectory X C. trajectory Y D. trajectory Z
A 25.0 cm long organ pipe is filled with air and is open at one end and closed at the other. The velocity of sound in air at 0 is 331 m/s. What is the frequency of the fifth mode?
is 331 m/s. What is the frequency of the fifth mode?
A. 1,550 Hz B. 1,750 Hz C. 2,320 Hz D. 2,720 Hz E. 3,170 Hz
Molecular clouds appear more transparent at longer wavelengths
Indicate whether the statement is true or false
A transformer is designed to step down the 120 V line voltage to 9 V. If there are 400 turns on the input coil, how many turns should there be on the output coil?