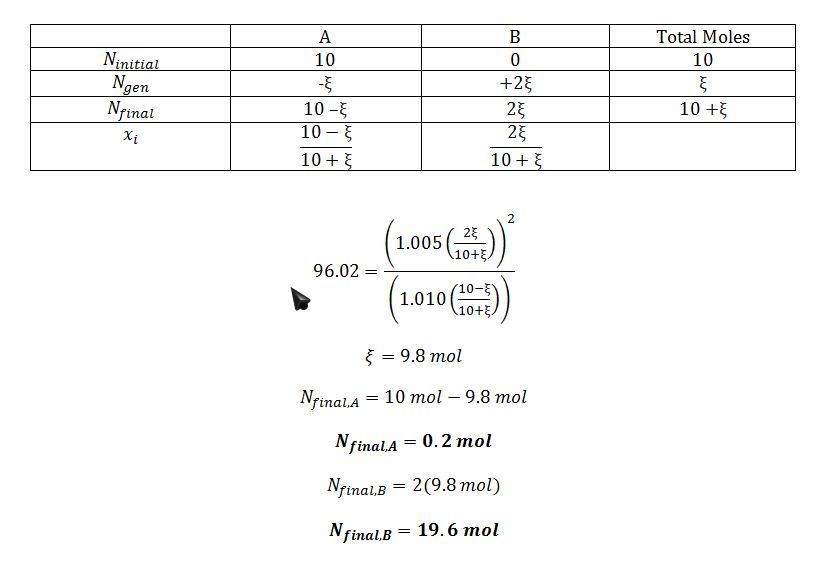
Determine Determine the contents of the reactor at equilibrium at 400 K, using the assumption that A and B form ideal solutions in the liquid phase.
A vessel initially contains 10 moles of pure liquid A at P=5 atm and T=400 K. The following reaction occurs isothermally and isobarically until equilibrium is achieved:
A ?2B
Thermochemical data on the two compounds in the liquid phase is presented in the following table. Values of G and H were obtained with a reference pressure of 1 bar:
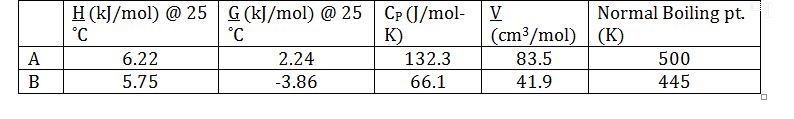
Apply equilibrium relationship between K_T and the mixture fugacities of the compounds:
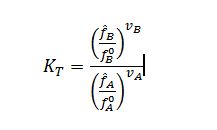 Where f ?_A=??_A x?_A f_A and f ?_B=??_B x?_B f_B are the mixture fugacities of A and B respectively, and f_A^0 and f_B^0 are the pure component fugacities of A and B respectively in their reference states (P = 100 kPa), and v_A and v_B are the stoichiometric coefficients of compounds A and B respectively. Since the liquid is being modeled as an ideal solution, the activity coefficients in each mixture fugacity expression are equal to 1:
Where f ?_A=??_A x?_A f_A and f ?_B=??_B x?_B f_B are the mixture fugacities of A and B respectively, and f_A^0 and f_B^0 are the pure component fugacities of A and B respectively in their reference states (P = 100 kPa), and v_A and v_B are the stoichiometric coefficients of compounds A and B respectively. Since the liquid is being modeled as an ideal solution, the activity coefficients in each mixture fugacity expression are equal to 1:
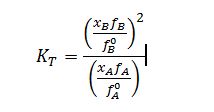
Apply Poynting correction to liquid fugacities:
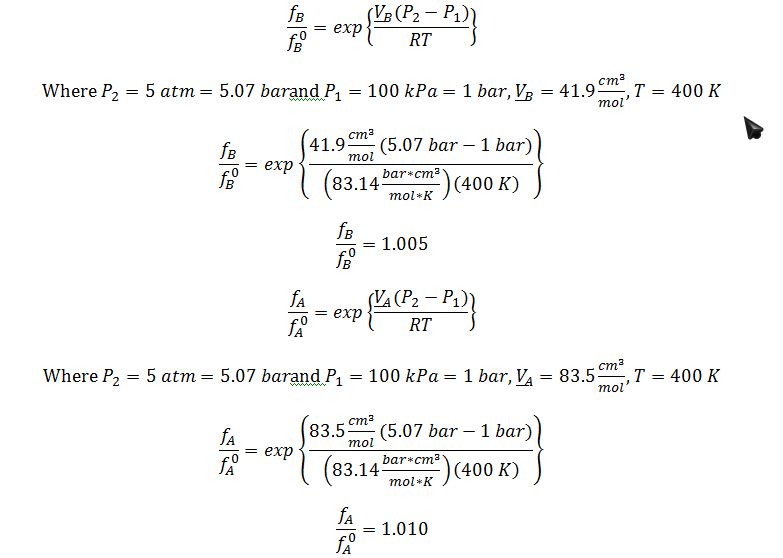
Substitute Poynting corrections into equilibrium relationship (this result also demonstrates that an assumption that the Poynting correction factor is negligible would be justified):
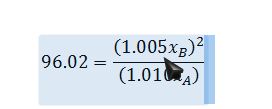
Substitute Poynting corrections into equilibrium relationship (this result also demonstrates that an assumption that the Poynting correction factor is negligible would be justified):
You might also like to view...
As an early biotechnology process, humans used sinews to do which of the following?
a. make wall drawings c. tie tools together b. bind animal hooves d. create clothing
Anthony is comparing the quality of an $800 personal computer to one that costs $1,634
This is an example of the ________ of quality.
a. relative nature b. absolute nature c. conformance dimension d. price dimension e. None of these
During the design and development stages of a remote meter reading system for residential electricity use, the two engineers working on the project for the City of Forest Ridge noted something different than they expected. The first, an electrical/ software engineer, noted that their city liaison staff member provided all the information on the software options, but only one option, the one from Lorier Software, was ever discussed and detailed. The second designer, an industrial systems engineer, further noted that all the hardware specifications provided to them by this same liaison all came from the same distributor, namely, Delsey Enterprises. Coincidently, at a weekend family picnic for city employees, to which the engineers had been invited, they met a couple named Don Delsey and
Susan Lorier. Upon review, they learned that the man Don is the son-in-law of the city liaison and Susan is his step-daughter. Based on these observations, before they proceed further with development of the system, what should the two engineers do, if anything, about their suspicions that the city liaison person is trying to bias the design to favor the use of his relative’s software and hardware businesses? What will be an ideal response?
____________________ allows us to figure out approximately what the answer should be.
Fill in the blank(s) with the appropriate word(s).