When a sample of water at 0.0°C is cooled to -36.0°C and freezes in the process, 935,000 kJ of heat is liberated
What is the mass of this sample of water? For water LF = 334,000 J/kg, LV = 2.256 × 106 J/kg, and the specific heat of ice is 2050 J/kg ? °C.
A) 2290 kg
B) 1145 kg
C) 2800 kg
D) 12,700 kg
A
You might also like to view...
What was Miller and Urey trying to replicate in their experiment?
a. the formation of cell b. the formation of ozone c. the formation of photosynthesis d. the formation of organic molecules e. the formation of liquid water
The Viking spacecraft searched for life on Mars by looking for the effects of life on the upper atmosphere
a. True b. False Indicate whether the statement is true or false
Consider the right-going wave and the possible left-going waves displayed in problem Q2T.3. For which of the choices will there be an instant where the combined wave is a rectangular wave as shown below? 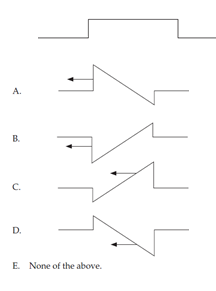
A. A B. B C. C D. D E. None of the above
A plasma will conduct electricity
a. True b. False Indicate whether the statement is true or false