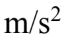A sealed container holds 0.020 moles of ideal nitrogen (N2 ) gas, at a pressure of 1.5 atm and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol
How many molecules of nitrogen are in the container? (R = 8.31 J/mol ? K, 1 atm = 101 kPa)
A) 1.5 × 1021 mol
B) 3.0 × 1021 mol
C) 6.0 × 1021 mol
D) 1.2 × 1022 mol
E) 2.4 × 1022 mol
D
You might also like to view...
What is the potential difference across C2 when C1 = 5.0 ?F, C2 = 15 ?F, C3 = 30 ?F, and V0 = 24 V?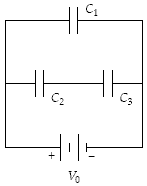
A. 21 V B. 19 V C. 16 V D. 24 V E. 8.0 V
A 5-µF, a 7-µF, and an unknown capacitor CX are connected in series between points a and b. What do you know about the equivalent capacitance Cab between a and b? (There could be more than one correct choice.)
A) 5 µF < Cab < 7 µF B) Cab < CX C) Cab > 12 µF D) Cab < 5 µF E) 5 µF < Cab < 12 µF
If we wished to construct a "tank circuit" where electric charge originally stored on a capacitor flows through an inductor, then back again, what value of inductance should we place in series with a fully-charged 100 ?F capacitor to get the circuit to resonate at 60.0 Hz?
A small golden statue falls from a height of 8.2 m above the top surface of a pillow. If the acceleration of the statue upon striking the pillow is constant, what is the value of the acceleration if the pillow compresses by 3.9 cm as the statue comes to rest?
A. 2100 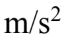
B. 21 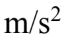
C. 160 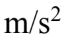
D. 210