What is the partial molar enthalpy (in the proper units) of component water (at this T and P) at the following mole fractions?
Suppose that the liquid molar enthalpy (H) for a binary mixture of hydrogen fluoride (1) and water (2) has been fitted to the following functional form (at 20°C and 1 atm) :
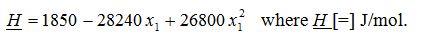
A) x1 = 0.4
B) x1 = 0.0
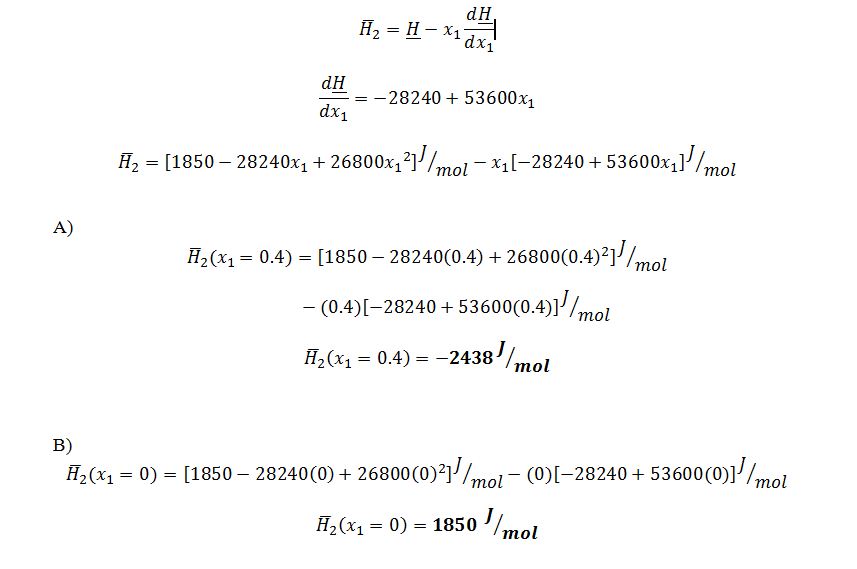
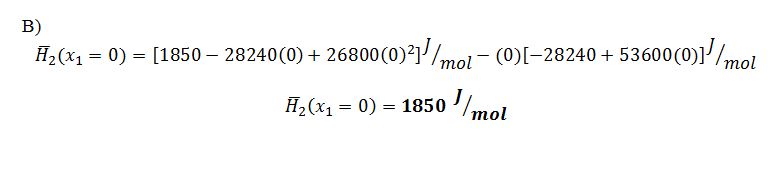
You might also like to view...
What part of a solution is the substance present in a solution in the largest amount?
A) Soluble B) Solvent C) Insoluble D) Solute
What does the term bobtailing mean?
A. Driving any truck unloaded B. Driving a truck-train combination C. Driving a tractor uncoupled from a trailer D. Driving by weaving in and out of lanes
Computers have already revolutionized the way decisions are made on the farm
Indicate whether the statement is true or false
Why is a library of piping symbols important when drawing with CAD?
What will be an ideal response?