How much energy, in kilojoules, is released or absorbed from the reaction of one mole of nitrogen, N2, with three moles of molecular hydrogen, H2, to form two moles of ammonia, NH3? H-N (bond energy: 389 kJ/mol)H-H (bond energy: 436 kJ/mol)N 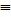 N (bond energy: 946 kJ/mol) N
N (bond energy: 946 kJ/mol) N 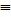 N + H-H + H-H ? NH3 + NH3
N + H-H + H-H ? NH3 + NH3
A. ?80 kJ/mol released
B. +80 kJ/mol absorbed
C. +899 kJ/mol absorbed
D. ?993 kJ/mol released
Answer: A
You might also like to view...
The principle of lateral continuity says that if a fault occurs in a sequence of sedimentary rocks, the fault is older than the rocks
Indicate whether the statement is true or false
Fossil fuels are generated by processes deep in the Earth's interior
Indicate whether the statement is true or false
Two tectonic plates collide at a transform plate boundary.?
Answer the following statement true (T) or false (F)
In asexual reproduction, ________
A) the offspring develop from a different zygote B) the offspring are made up of portions of each of the parent's DNA C) the offspring are genetically related but also genetically distinct D) the offspring are genetically unique E) the offspring have identical DNA