Kirchhoff was the first to describe qualitatively the types of spectra we observe from luminous objects. Describe the three types and their sources
What will be an ideal response?
His first law has dense hot objects creating a continuous spectrum. In the second law, a low density hot gas gives off bright emission lines. In the third law, a cool thin gas in front of a continuous source will absorb some of the energies, creating dark lines in the spectrum.
You might also like to view...
The method of spectroscopic parallax cannot be applied to stars beyond about 100 pc
a. True b. False Indicate whether the statement is true or false
Ideal Gas Law: A 0.40-m3 gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. What is the pressure of the gas? (R = 8.31 J/mol ? K, 1 atm = 101 kPa)
A. 42 atm B. 37 atm C. 32 atm D. 27 atm E. 22 atm
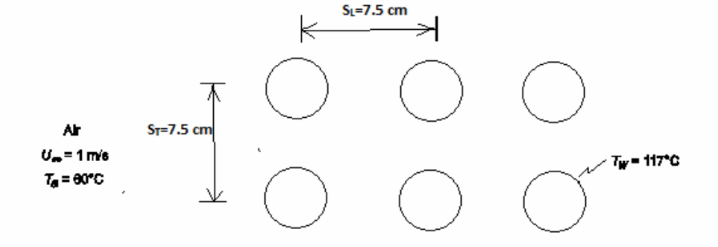
Reconsider the heat exchanger of Problem 6.38 with an inline tube arrangement, where the centerlines of the tubes are spaced 7.5 cm apart, both in the longitudinal and transverse directions. Compare the results with those for the staggered tube arrangement and comment upon the difference. Also, which of the two arrangements is expected to have a larger pressure drop?
GIVEN
• Air flow through a tube bank
• Tube spacing (S) = 7.5 cm = 0.075 m
• Air temperature (Ta) = 60°C
• Air velocity (Us) = 1 m/s
• Tube outside diameter (D) = 6 cm = 0.06 m
• Tube wall temperature (Tw) = 117°C FIND
• The average heat transfer coefficient ( ch )
• Steady state
SKETCH
By operating a reversible heat engine with an ideal gas as the working substance in a Carnot cycle and measuring the ratio Qc/Qh, we can calculate
a. n, the number of moles of the ideal gas. b. the ratio Vc/Vh of the volumes of the ideal gas. c. the ratio Pc/Ph of the pressures of the ideal gas. d. the ratio PcVc/PhVh of the products of volumes and pressures of the ideal gas. e. the value of Avogadro's number.