Solve.The rate R of a chemical reaction (in grams per liter per minute) is given by the expression R = 0.79C2/5, where C is the concentration of the reactant in grams per liter. On log-log paper, graph R as a function of C for C = 0.001 g/L to C = 0.1 g/L.
What will be an ideal response?
Graph on separate log-log paper.
Mathematics
You might also like to view...
Solve the problem. Round rates to the nearest tenth of a percent and dollar amounts to the nearest cent.A store sells an item for $240 each. If this is a 56.1% markup on the selling price, find the equivalent markup percent on cost.
A. 240% B. 35.9% C. 81.9% D. 127.8%
Mathematics
Divide.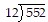
A. 46 R 4 B. 47 R 2 C. 47 R 5 D. 46
Mathematics
Write the exponential equation in logarithmic form.4-2 = 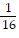
A. log1/16 4 = -2
B. log4 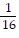 = -2
= -2
C. log4 -2 = 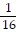
D. log-2 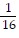 = 4
= 4
Mathematics
Simplify the expression. Write the result without using negative exponents. (Assume all variables represent nonzero real numbers.)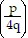 -4
-4
A. 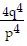
B. 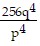
C. 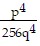
D. - 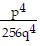
Mathematics