Consider the Bohr model for the hydrogen atom. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ? s)
(a) How much energy (in eV) is needed to cause a transition of an electron from the second excited state to the third excited state?
(b) What wavelength photon just has enough energy to initiate the transition in (a)?
(a) 0.661 eV (b) 1.88 μm
You might also like to view...
Density: Substance A has a density of 3 g/cm3 and substance B has a density of 4 g/cm3. In order to obtain equal masses of these two substances, what must be the ratio of the volume of A to the volume of B?
A. 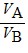 =
= 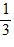
B. 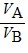 =
= 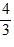
C. 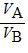 =
= 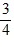
D. 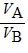 =
= 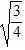
E. 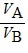 =
= 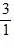
A car travels in a flat circle of radius R. At a certain instant the velocity of the car is 24 m/s west, and the total acceleration of the car is 2.5 m/s2 53° north of west. Which of the following is correct?
A. R = 0.29 km, and the car's speed is increasing. B. R = 0.23 km, and the car's speed is decreasing. C. R = 0.23 km, and the car's speed is increasing. D. R = 0.29 km, and the car's speed is decreasing. E. R = 0.29 km, and the car's speed is constant.
Abby throws a ball straight up and times it. She sees that the ball goes by the top of a flagpole after 0.50 s and reaches the level of the top of the pole after a total elapsed time of 4.10 s
What was the speed of the ball at as it passed the top of the flagpole? A) 6.40 m/s B) 16.2 m/s C) 17.6 m/s D) 29.0 m/s E) 33 m/s
On a giant rotating turntable are two clocks, one at the center and the other at the rim.The clock that runs slower is at
A) the center of the turntable. B) the rim of the turntable. C) either of these