Two moles of an ideal gas are placed in a container of adjustable volume. When measurements are made
A. the pressure is inversely proportional to the volume at constant temperature.
B. the temperature is directly proportional to the volume at constant pressure.
C. the temperature is directly proportional to the pressure at constant volume.
D. all the statements above are found to be correct.
E. only statements (a) and (b) are found to be correct.
Answer: D
You might also like to view...
A 4-cm-diameter cylindrical enclosure with black surfaces, as shown in the accompanying sketch, has a 2-cm hole in the top cover. Assuming the walls of the enclosure are at the same temperature, determine the percentage of the total radiation emitted from the walls that escapes through the hole in the cover.
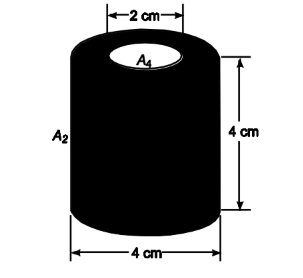
GIVEN
• Cylindrical enclosure of black surfaces shown above
• Cylinder diameter (D) = 4 cm = 0.04 m
• Diameter of hole in top (Dh) = 2 cm = 0.02 m
FIND
• The percentage of the total radiation emitted from the wall which will escape through the hole in the cover (Fe4).
ASSUMPTIONS
• The walls of the enclosure are at the same temperature
A building made with a steel structure is 650 m high on a winter day when the temperature is 0° F. How much taller (in cm) is the building when it is 100° F? (The linear expansion coefficient of steel is 11 × 10^?6 (°C)?1.)
a. 71 b. 36 c. 40 d. 46 e. 65
What is 0.2052/3, to the correct number of significant figures?
A) 0.348 B) 0.35 C) 0.3 D) 0.3477
In this video, angular momentum is only approximately conserved. This is because
Choose one: A. there are small external forces acting—friction and air resistance, for example. B. no quantities are ever really conserved. C. the person on the platform is exerting a force when she pulls her arms in and pushes them out. D. the measurement of angular momentum depends on your reference frame.