How does fractional distillation work?
A) It takes advantage of the different boiling points of molecules to separate them.
B) It takes advantage of the different weight of molecules to separate them.
C) It uses the fraction of carbon in the isomers to separate the molecules.
D) It takes advantage of the different melting points to separate molecules.
E) none of the above
Answer: A
You might also like to view...
The cosmological redshifts of galaxies are Doppler shifts
a. True b. False Indicate whether the statement is true or false
If our eyes were sensitive only to X rays, the world would appear __________.
A. gray, black, and white like a medical X ray B. brighter than normal because X rays carry more energy than visible light photons C. dark because X-ray light does not reach Earth’s surface D. green, yellow, and orange, because those are the colors of X rays
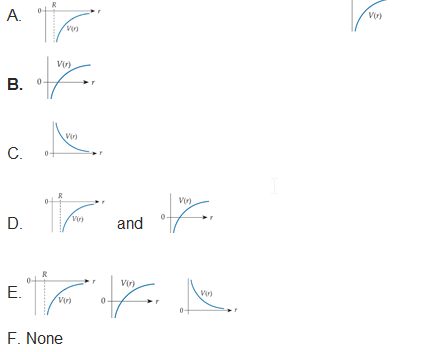
Which of the lettered graphs below might also legitimately describe the potential energy for the same interaction (between the same two particles!) whose potential energy function is shown in the “original” graph in the upper left? In each case, the vertical axis is energy (increasing upward) and that axis crosses the horizontal axis where r = 0.
A 2-qt bottle of soda is on sale for $1.29. What should be the price of a 2-Liter bottle of the same soda to yield the same value?
What will be an ideal response?