Because HF, hydrogen fluoride, is a covalent gaseous molecule at room temperature, we might reasonably expect that at room temperature HCl, hydrogen chloride, is
a. a covalent gaseous molecule.
b. a covalent solid.
c. a metallic gas.
d. a metallic solid.
e. an ionic solid.
a
You might also like to view...
Ohm's Law: For the graph shown in the figure, what physical quantity does the slope of the graph represent for ohmic material?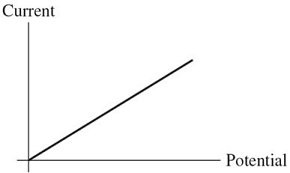
A. power B. resistivity C. 1/(resistivity) D. resistance E. 1/(resistance)
Answer the following statement(s) true (T) or false (F)
1. Suppose we add a certain amount of energy to a quanton in a box, increasing its energy from 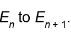 . Adding the same amount of energy again will increase its energy to
. Adding the same amount of energy again will increase its energy to 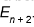 .
.
2. Suppose we add a certain amount of energy to a simple quantum harmonic oscillator, increasing its energy
from 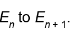 . Adding the same amount of energy again will increase its energy to
. Adding the same amount of energy again will increase its energy to 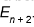
Both the refrigerator and the heat pump take in heat and expel it at a higher temperature. Why don't they have the same Coefficient of Performances?
What will be an ideal response?
On a warm day a pool of water transfers energy to the air as heat and freezes. This is a direct violation of:
A. the zeroth law of thermodynamics B. the first law of thermodynamics C. the second law of thermodynamics D. the third law of thermodynamics E. none of the above