Molar Specific Heats: A rigid container is filled with 4.0 mol of air with CV = 2.5R. How much does the internal (thermal) energy of the air change if its temperature rises from 18°C to 448°C? (R = 8.31 J/mol ? K)
A. 35,800 J
B. 430 J
C. 3580 J
D. 8940 J
Answer: A
You might also like to view...
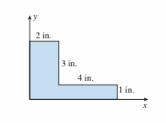
The centroid of the L-shaped segment shown in the figure below is at coordinates (x, y) (in.):
(A) 2.0, 1.75
(B) 3.0, 1.50
(C) 2.0, 1.50
(D) 2.5, 1.25
Which Greek school of thought believed that life on Earth was unique?
A) the Aristotelians B) the Platonists C) the stoicists D) the atomists
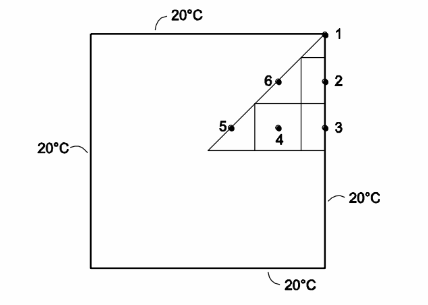
A 1-cm-thick, 1-m-square steel plate is exposed to sunlight and absorbs a solar flux of 800 W/m2. The bottom of the plate is insulated, the edges are maintained at 20°C by water-cooled clamps, and the exposed face is cooled by a convection coefficient of 10 W/(m2 K) to an ambient temperature of 10°C. The plate is polished to minimize reradiation. Determine the temperature distribution in the plate using a node spacing of 20 cm. The thermal conductivity of the steel is 40 W/(m K).
GIVEN
Square plate with water-cooled edges exposed to solar flux
FIND
(a) Temperature distribution in the plate
ASSUMPTIONS
(a) Neglect temperature gradients through the plate thickness
SKETCH
A man in a gym is holding an 8.0-kg weight at arm's length, a distance of 0.55 m from his shoulder joint. What is the torque about his shoulder joint due to the weight if his arm is held at 30° below the horizontal?
A) 22 N ? m B) 2.2 N ? m C) 4.4 N ? m D) 13 N ? m E) 37 N ? m