From a molecular point of view, explain why evaporation is a cooling process. What cools?
What will be an ideal response?
Answer: Evaporation is the change of phase from liquid to gas. The molecules in the liquid most prone to make the transition and break through the surface to become a gas are the faster and more energetic molecules. So the molecules that evaporate are the faster, or more energetic. With selective removal of the more energetic molecules, a greater proportion of slower ones remain in the liquid. Hence the liquid is cooled.
You might also like to view...
One mole of hydrogen gas and 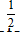 mole of helium gas are mixed in a container and maintained at a fixed temperature T. How does the total pressure PH exerted by the hydrogen molecules compare to the total pressure PHe exerted by the helium molecules? Note that a molecule (atom) of helium has twice the mass of an H2 molecule.
mole of helium gas are mixed in a container and maintained at a fixed temperature T. How does the total pressure PH exerted by the hydrogen molecules compare to the total pressure PHe exerted by the helium molecules? Note that a molecule (atom) of helium has twice the mass of an H2 molecule.
A. PH = 2PHe
B. PH = 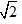 PHe
PHe
C. PH = PHe
D. PH =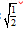 PHe
PHe
E. PH = 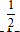 PHe
PHe
F. Other (specify)
Condensation in the solar nebula probably led to the formation of
a. icy grains beyond the present orbit of Jupiter. b. metallic grains near the present orbit of Mercury. c. silicate grains near the present orbit of Earth. d. all of the above e. none of the above
Exploration of this part of the atmosphere was conducted in the nineteenth century with hot-air balloons
a. Stratosphere b. Troposphere c. Mesosphere d. Thermosphere
Power: A 200-? resistor is rated at 1/4 W. What is the maximum voltage that can safely be connected across it?
A. 0.71 V B. 7.1 V C. 50 V D. 0.25 V