The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 calories of heat. What is the mass of the sample? (Specific heat of water is 1.0 cal/g °C)
A) 56.9 g
B) 622 g
C) 212 g
D) 310 g
B) 622 g
C) 212 g
D) 310 g
Ans: 212g
Physics & Space Science
You might also like to view...
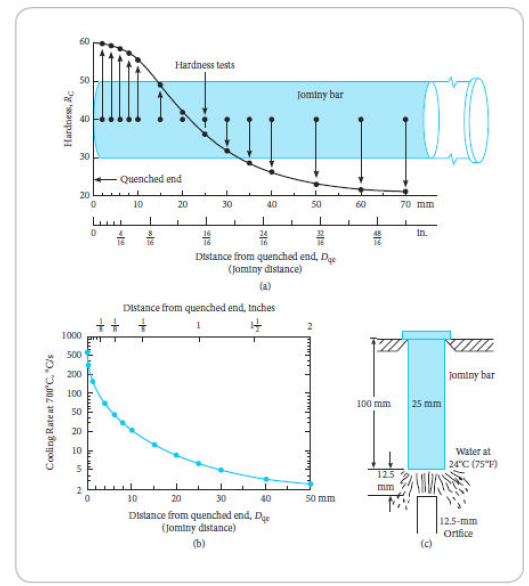
Suggest a surface cooling rate to achieve a hardness of Rockwell C 55 instead of 59, referring to Figure 3-23 and Figure 3-23b.
FIG 3-23
Jominy end-quench test
Physics & Space Science
Determine the equivalent capacitance of the combination shown when C = 15 mF
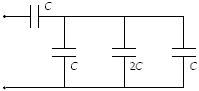
a.
20 mF
b.
16 mF
c.
12 mF
d.
24 mF
e.
75 mF
Physics & Space Science
How do we learn about Earth’s core, mantle, and crust?
a. magnetic field studies b. seismic waves c. x-ray imaging d. all of the above e. a and b
Physics & Space Science
What is light pollution?
What will be an ideal response?
Physics & Space Science