Oxygen gas is forced into an aquarium at 1 atm and 25°C, and the oxygen bubbles are observed to rise to the free surface in 4 s. Determine the penetration depth of oxygen into water from a bubble during this time period.
What will be an ideal response?
An aquarium is oxygenated by forcing oxygen to the bottom of it, and letting the oxygen bubbles rise. The penetration depth of oxygen in the water during the rising time is to be determined.
Assumptions 1 Convection effects in the water are negligible. 2 The pressure and temperature of the oxygen bubbles remain constant.
Properties The mass diffusivity of oxygen in liquid water at 298 K is DAB = 2.4 ×10-9 m2 /s (Table 14-3b).
Analysis The penetration depth can be determined directly from its definition (Eq. 14-38) to be
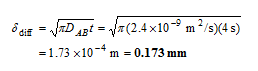
Therefore, oxygen will penetrate the water only a fraction of a milimeter.
You might also like to view...
Euthanasia rates have dropped significantly since the 1980s
Indicate whether the statement is true or false
Technician Asays the conventional theory of current flow states that current flows positive to negative. Technician B says current flows randomly. Who is correct?
A. Technician A only B. Technician B only C. Both Technician A and B D. Neither Technician A nor B
Give the correct order for progressively reducing the size of soil lumps.
A. Moldboard plow, disk harrow, harrow B. Ripper, harrow, chisel plow C. Disk harrow, chisel plow, lister D. Cultivator, harrow, lister
Valves installed at the highest point in the system are called _____
a. check valves b. bleeder valves c. top valves d. traps