What is the Pauli exclusion principle?
a. Only two electrons can be in the same state (have the same quantum numbers).
b. No two electrons can be in the same state (have the same quantum numbers).
c. Electrons repel each other.
d. No two elements can have electrons in the same state (with the same quantum numbers).
e. No two electrons can occupy the same shell.
b
You might also like to view...
Force on a Moving Charge: Three particles travel through a region of space where the magnetic field is out of the page, as shown in the figure. What are the signs of the charges of these three particles?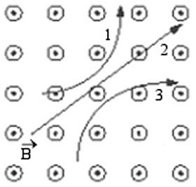
A. 1 is neutral, 2 is negative, and 3 is positive. B. 1 is neutral, 2 is positive, and 3 is negative. C. 1 is positive, 2 is neutral, and 3 is negative. D. 1 is positive, 2 is negative, and 3 is neutral. E. 1 is negative, 2 is neutral, and 3 is positive.
Which of the following shapes is not an allowed trajectory of an object orbiting a star? (Assume no gas or dust is affecting the object's orbit.)
A) a hyperbola B) a parabola C) a spiral D) an ellipse
The critical angle for a particular type of glass is 39.0°. What is the index of refraction of the glass?
A) 1.52 B) 1.55 C) 1.57 D) 1.59 E) 1.50
As a water wave approaches a shoreline, wave speed
A) increases. B) decreases. C) is unchanged. D) and wavelength increase.