Consider a propane (1) + n-butane (2) system at 348.15 K. If you assume an ideal gas for this system, what will be the value of the mixture fugacity coefficient for propane when the composition of propane in the mixture is 46% by mole?
A. 0
B. 1
C. 0.46
D. 1.46
E. The pressure is required to make the calculation.
A. Incorrect. This number is not physically realistic.
B. Correct. Since this is an ideal gas, per the statement above, the mixture fugacity coefficient must be equal to 1.
C. Incorrect. The mixture fugacity coefficient is not equal to the composition.
D. Incorrect. Reread the problem statement, and review the definition of fugacity and the fugacity coefficient.
E. Incorrect. The mixture fugacity will be a function of pressure for an ideal gas, but not the fugacity coefficient.
?
You might also like to view...
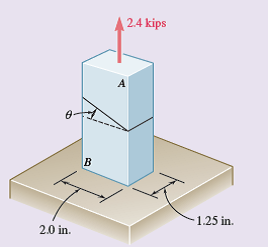
Problem 1.69 The two portions of member AB are glued together along a plane forming an angle ? with the horizontal. Knowing that the ulti¬mate stress for the glued joint is 2.5 ksi in tension and 1.3 ksi in shear, determine (a) the value of ? for which the factor of safety of the member is maximum, (b) the corresponding value of the factor of safety. (Hint: Equate the expressions obtained for the factors of safety with respect to the normal and shearing stresses.)
_________________________ chickens are raised on pasture and forage for insects with some supplementation of grains to their diets
Fill in the blank(s) with correct word
It is possible for a refrigerant to absorb heat at a low temperature and reject heat at a higher temperature.
Answer the following statement true (T) or false (F)
Altera uses different terms for Set and Reset in the DFF symbol. What are they?
What will be an ideal response?