Develop equations that may be solved to give the final temperature of the gas remaining in a tank after the tank has been bled from an initial pressure 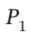 to a final pressure
to a final pressure  Known quantities are initial temperature, tank volume, heat capacity of the gas, total heat capacity of the containing tank,
Known quantities are initial temperature, tank volume, heat capacity of the gas, total heat capacity of the containing tank, 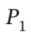 , and
, and 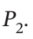 Assume the tank to be perfectly insulated.
Assume the tank to be perfectly insulated.
What will be an ideal response?
We can re-use the sketch from the last problem, with slight modifications:
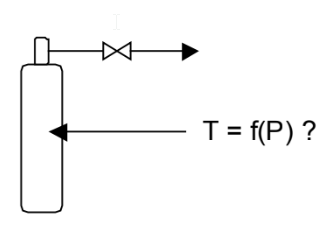
Our goal is to develop a relationship between the temperature in the tank and the pressure in the tank. We will write mole and energy balances on the tank. This time, however, the tank is insulated on the outside (so Q = 0) but the tank itself changes temperature with the gas. We will take as our control volume the tank and the gas inside the tank, up to a point just before the valve. Then the mole balance is
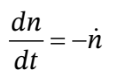
and the energy balance is
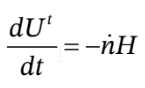
in the energy balance, Q = W = 0. 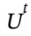 is the total internal energy of the system, which includes both the tank and the gas inside. So, we can write
is the total internal energy of the system, which includes both the tank and the gas inside. So, we can write 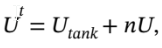 where
where 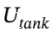 is the total internal energy of the tank and U is the molar internal energy of the gas. We can also write H = U + PV = U + RT.
is the total internal energy of the tank and U is the molar internal energy of the gas. We can also write H = U + PV = U + RT.
Subsituting these in gives
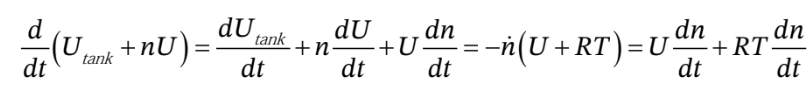
or
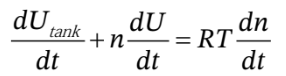
where I have gone ahead and expanded the derivative on the left hand side using the product rule, and have used the mole balance to substitute for 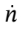 on the right-hand-side. Now, we can replace the internal energy terms with temperatures by noting that
on the right-hand-side. Now, we can replace the internal energy terms with temperatures by noting that 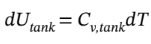 and
and 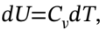 where
where 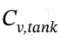 is the total heat capacity of the tank, and
is the total heat capacity of the tank, and 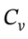 is the molar heat capacity of the gas. Canceling the term that appears on both sides of the
is the molar heat capacity of the gas. Canceling the term that appears on both sides of the
equation above and then substituting these gives
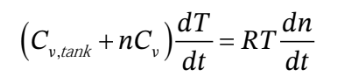 Rearranging this to put the T’s on one side and the n’s on the other, then integrating from the initial to final number of moles in the tank gives
Rearranging this to put the T’s on one side and the n’s on the other, then integrating from the initial to final number of moles in the tank gives
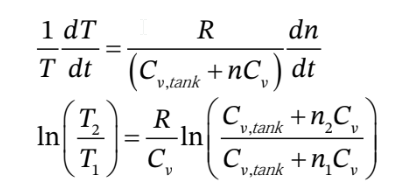
This gives us a relationship between the number of moles in the tank and the temperature, but we want a relationship between the pressure in the tank and the temperature. We can substitute for the number of moles in terms of the
temperature and pressure using the ideal gas law:
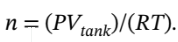 This gives
This gives
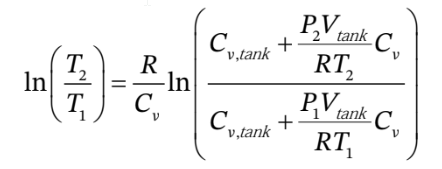
or
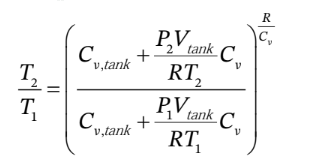
This could be solved numerically for 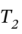 given all of the other information.
given all of the other information.
You might also like to view...
The _____________________ is a cycle of operation of a heat pump that is designed to defrost the outside coil during the heating operation
Fill in the blank(s) with the appropriate word(s).
Apple Computer wants to have $2.1 billion available 5 years from now in order to finance initial production of a device that applies IOT technology for home use. The company expects to set aside uniformly increasing amounts of money each year to meet its goal, starting with $100 million at the end of year 1. How much will the constant increase, G, have to be each year at a rate of return of 18% per year? Try your skill by using the Goal Seek tool to find the required gradient. Start the evaluation with G = $50 million per year.
What will be an ideal response?
A ____ is a cold-rolled angle attached to the concrete floor of the building that provides a continuous base to which the bottom of the sheeting panel can be attached.
A. base angle B. base-panel angle C. floor-panel angle D. sheeting angle
How is the type of service pressure of a recovery tank identified?
A) By the listed weight of the tank B) By the first numbers listed on the tank C) By the first number after the letters "DOT" D) By the last group of numbers on the tank