1.50 moles of an ideal monatomic gas are initially at a temperature of 317 K. If the gas gains 2730 J of heat and performs 780 J of work, what is its final temperature?
A) 359 K
B) 421 K
C) 526 K
D) 687 K
E) 756 K
B
You might also like to view...
The Oort Cloud is thought to be
A) the cloud of gas and dust from which our solar system formed. B) a cloud of debris that occasionally encounters the Earth, causing a meteor shower. C) the spherical cloud of comets and some larger icy bodies surrounding the outer solar system. D) a cloud of asteroids moving between the orbits of Mars and Jupiter. E) the material in the ecliptic plane that creates the zodiacal light.
An astronaut is piloting a spacecraft, which is in a circular orbit around Earth. A space station is ahead, on the same circular orbit. If he fires his rockets briefly to increase the forward speed of the rocket, what will happen?
What will be an ideal response?
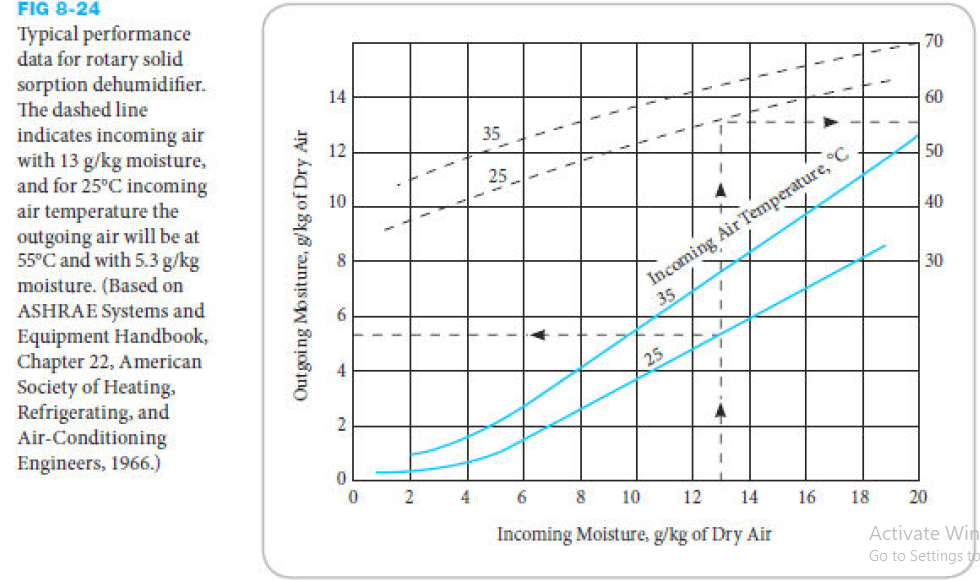
A rotary solid sorption dehumidifier is used to dehumidify air at 95ºF, 60% relative humidity. If the dehumidifier performance map is like that shown in Figure 8-24, estimate the outlet dry air temperature and its relative humidity. Then estimate the rate of water removed from 100 cfm of air.
If body P, with a positive charge, is placed in contact with body Q (initially uncharged), what will be the nature of the charge left on Q?
a. must be equal in magnitude to that on P b. must be negative c. must be positive d. must be greater in magnitude than that on P