A compound has the following properties at atmospheric pressure:
• Normal boiling point of 95 °C
• Specific enthalpy of vaporization of 900 J/g
• In the liquid phase, CP = 2.4 J/gK
• In the vapor phase, CP = 3.0 J/gK
Find the change in enthalpy when 500 grams of the compound are heated at atmospheric pressure from liquid at 50 °C to vapor at 150 °C.
1 = liquid at 50 °C and 1 atm
2 = saturated liquid at 95 °C and 1 atm
3 = saturated vapor at 95 °C and 1 atm
4 = vapor at 150 °C and 1 atm
Enthalpy is a state property, so:
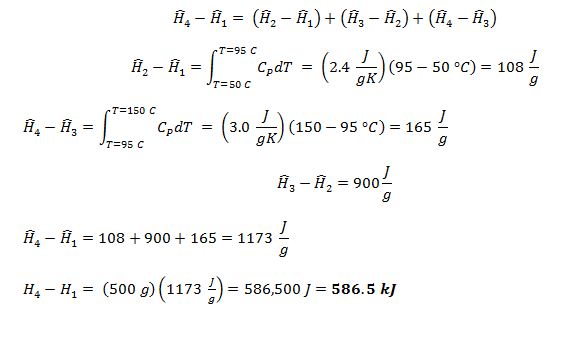
You might also like to view...
ASTM specifications classify masonry mortar into Type M, Type S, Type N, and Type O. Which of these types is recommended for use in non-load-bearing interior walls?
What will be an ideal response?
The isotope of hydrogen that makes up heavy water is tritium
Indicate whether the statement is true or false.Which of the following statements regarding photovoltaic cells on a green roof is MOST accurate?
A. The energy feed from photovoltaic cells can be easily shut off. B. Photovoltaic cells are readily identifiable during fire fighting operations. C. A roof containing photovoltaic cells may not have space for rooftop access and ventilation. D. Photovoltaic cells are unable to store and generate electric power when the electric power to a building is shut off.
An electrical circuit using 12 volts and 5 amps of current would have a resistance of:
A. 2.4 ohms B. .2 ohms C. 1 ohm D. 12 ohms