An unknown gas occupies a volume of 0.075 m3, has a pressure of 0.500 MPa, and a temperature of 47°C. You determine that the gas is either CO2, O2, N2, or CH4. Using the volume, pressure, and temperature given, determine the mass that would be expected for these four gases. If you measure the mass to be 0.441 kg, which of the four gases is the unknown gas likely to be?
Given: V = 0.075 m3; P = 500 kPa; T = 47°C = 320 K
What will be an ideal response?
Using the ideal gas law, the mass can be found from m = PV/RT
Below is a table containing the gases, their R values, and the calculated masses.
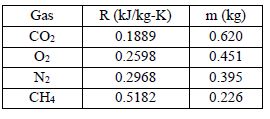
The measured mass of 0.441 kg means the gas is most likely O2.
You might also like to view...
What are five key points to consider when selecting a site for planting of bramble
fruits? Of these points, which ones are most important and why?
What will be an ideal response?What is the function of aerators?
What will be an ideal response?
Exhaust hoses should be used because one of the exhaust gases can be deadly in high concentrations. This gas is _________
A) Oxides of nitrogen (NOx) B) Hydrocarbons (HC) C) Carbon monoxide (CO) D) Carbon dioxide (CO2)
In a linear circuit the signal at the output is bad. The testing approach that would reduce the number of measurements is to
A) Measure the signal at each block output starting at the output. B) Measure the input and then check the output of each block starting from the input. C) Make the first measurement at the output of the block half way between the input and output. D) None of the above.