A fixed amount of ideal gas is held in a rigid container that expands negligibly when heated. At 20°C the gas pressure is p. If we add enough heat to increase the temperature from 20°C to 40°C, the pressure will be
A) impossible to determine since we do not know the number of moles of gas in the container.
B) greater than 2p.
C) less than 2p.
D) equal to 2p.
E) impossible to determine since we do not know the volume of gas in the container.
Answer: C
You might also like to view...
Which of the following statements about the arrangement of movable objects in a space is true?
a. The placement of movable objects can decrease the amount and type of interaction in a space b. The construction of movable objects such as chairs can impact people's willingness to remain in a space, c. The placement of movable objects can increase the amount and type of interaction in a space. d. All of these statements are true:
Answer the following statement(s) true (T) or false (F)
1. 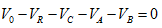
2. 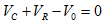
3. 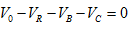
Higher mass protostars enter the main sequence:
A) faster and at a higher luminosity and temperature. B) faster and at a lower luminosity and temperature. C) slower and at a higher luminosity and temperature. D) slower and at a lower luminosity and temperature. E) at the same rate, but at a higher luminosity and temperature.
M31 in Andromeda is a bigger version of our Galaxy, and the largest member of the Local Group
Indicate whether the statement is true or false