Latent Heats: When a sample of water at 0.0°C is cooled to -36.0°C and freezes in the process, 935,000 kJ of heat is liberated. What is the mass of this sample of water? For water LF = 334,000 J/kg, LV = 2.256 × 106 J/kg, and the specific heat of ice is 2050 J/kg ? C°.
A. 2290 kg
B. 1145 kg
C. 2800 kg
D. 12,700 kg
Answer: A
You might also like to view...
Motional Emf: A conducting rod whose length is ? = 27.0 cm is placed on frictionless U-shaped metal rails that is connected to a lightbulb having a resistance of 5.00 ? as shown in the figure. The rails and the rod are in the plane of the page. A constant uniform magnetic field of strength 1.20 T is applied perpendicular to and out of the paper. An external applied force moves the rod to the right with a constant speed. At what speed should the rod be pulled so that the lightbulb will consume energy at a rate of 1.10 W?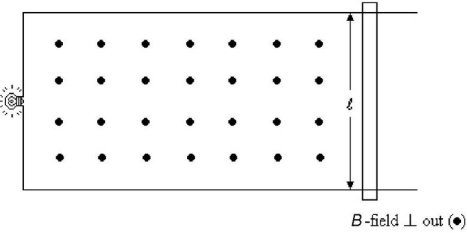
A. 2.00 m/s B. 3.50 m/s C. 4.26 m/s D. 6.00 m/s E. 7.24 m/s
Why does quartz sandstone have a high porosity and a high permeability?
What will be an ideal response?
A watch dial that continues to glow after a week in the dark is almost certainly
A) fluorescent. B) phosphorescent. C) polarized. D) radioactive.
The side of a cube is increased by 5%. The percentage increase of the surface area of the cube is
A. 5%. B. 10%. C. 12%. D. 16%. E. 18%.