Which statement below regarding the First Law of Thermodynamics is most correct?
a. A system can do work externally only if its internal energy decreases.
b. The internal energy of a system that interacts with its environment must change.
c. No matter what other interactions take place, the internal energy must change if a system undergoes a heat transfer.
d. The only changes that can occur in the internal energy of a system are those produced by non-mechanical forces.
e. The internal energy of a system cannot change if the heat transferred to the system is equal to the work done by the system.
e
You might also like to view...
Energy Conservation With Conservative Forces: When you throw a pebble straight up with initial speed V, it reaches a maximum height H with no air resistance. At what speed should you throw it up vertically so it will go twice as high?
A. 16V
B. 8V
C. 4V
D. 2V
E. 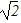 V
V
At what speed would a proton need to circle the Earth at a height of 1000 km above the equator if the Earth's magnetic field is horizontal and directed north-south, with an intensity of 4.00 × 10?8 T? (The radius of the Earth is 6400 km and the charge and mass of the proton are q = 1.60 × 10?19 C and mp = 1.67 × 10?27 kg. Ignore relativistic corrections.)
What will be an ideal response?
Stratocumulus clouds belong to the ______________ cloud family
Fill in the blank(s) with correct word
As a water wave approaches a shoreline, wave speed
A) increases. B) decreases. C) is unchanged. D) and wavelength increase.