One cubic meter of an ideal gas at 600 K and 1000 kPa expands to five times its initial volume as follows:
What will be an ideal response?
(a) By a mechanically reversible, isothermal process.
(b) By a mechanically reversible, adiabatic process.
(c) By an adiabatic, irreversible process in which expansion is against a restraining pressure of 100 kPa.
For each case calculate the final temperature, pressure, and the work done by the gas. Take 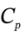 = 21
= 21 
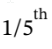
a. For isothermal expansion, the final temperature is, by definition, the initial temperature of 600 K. The final pressure is 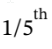 of the initial pressure, or 200 kPa. The work done on the gas is W = -
of the initial pressure, or 200 kPa. The work done on the gas is W = - 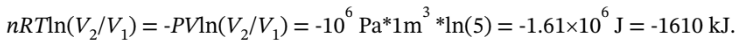 The work done by the gas is 1610 kJ, and the heat flow into the gas is 1610 kJ.
The work done by the gas is 1610 kJ, and the heat flow into the gas is 1610 kJ.
b. For the adiabatic process, the final pressure is given by 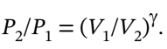 The heat capacity ratio is
The heat capacity ratio is 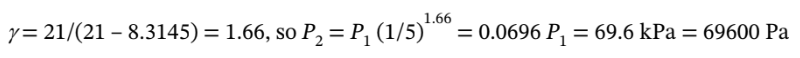
The final temperature is given by 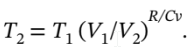
where 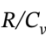 = 8.3145/(21 – 8.3145) = 0.6554,
= 8.3145/(21 – 8.3145) = 0.6554,
so 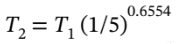 = 0.348
= 0.348 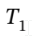 =0.348*600 K = 208.9 K.
=0.348*600 K = 208.9 K.
The work done on the gas can be computed as
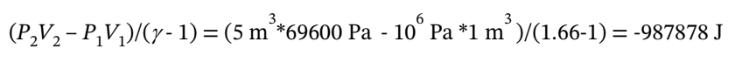
= -987.87 kJ. The work done by the gas is equal to 987.87 kJ.
c. In this case, the work is done against a constant resisting pressure of 100 kPa, so the total work done on the gas is just 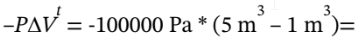 -400000 J = -400 kJ. This is a lot less than in the reversible case in part (b). In general, for a closed system,
-400000 J = -400 kJ. This is a lot less than in the reversible case in part (b). In general, for a closed system, 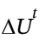 = Q + W. In this case, the process is adiabatic, so Q = 0 and we have
= Q + W. In this case, the process is adiabatic, so Q = 0 and we have 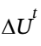 = W = -400 kJ. We also know that for a closed system,
= W = -400 kJ. We also know that for a closed system, 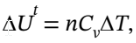 so we can get the
so we can get the
final temperature from
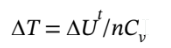
We can get n from the initial pressure, volume, and temperature, to be
n = PV/RT = (1000000*1)/(8.314*600) = 200.5 mol, so
?T = -400000 J/ (200.5 mol * (21 – 8.3145) J 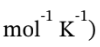 = -157.3 K
= -157.3 K
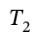 = 600 K – 157.3 K = 442.7 K
= 600 K – 157.3 K = 442.7 K
The gas doesn’t cool off nearly as much as in (b) because it doesn’t do nearly as much work. The final pressure is
P = nRT/V = 200.5*8.3145*442.7/5 = 147605.39 Pa = 147.61 kPa
You might also like to view...
A ____________________ is equal to one billionth of a gram
Fill in the blank(s) with correct word
When looking for the quality of materials and methods of construction for a floor assembly, you would refer
to the _____. a) specifications b) foundation plan c) floor plan d) structural drawings
Sometimes a ring gear needs to be heated to install it. This is one method of heating that should NOT be used:
a. torch b. induction heater c. oil d. heat gun
List the physics principles that make heat pump operation possible
What will be an ideal response?