Using the ideal gas law and the compressibility factor, determine the temperature of oxygen gas at a specific volume of 0.010 m3/kg and pressures of (a) 5,000 kPa, (b) 10,000 kPa, and (c) 20,000 kPa.
Given: v = 0.010 m3/kg, O2
What will be an ideal response?
For O2, R = 0.2598 kJ/kg-K
The temperature will be found by the following means:
Ideal Gas Law: T = Pv/R
Compressibility factor: T = Pv/ZR where Z is found from the compressibility charts,
using the critical properties of oxygen: Tc = 154.8 K, Pc = 5,080 kPa .
The reduced temperature is PR = P/Pc = 1.57
The pseudo-reduced specific volume is
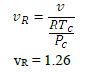
The results for the temperature are as follows:
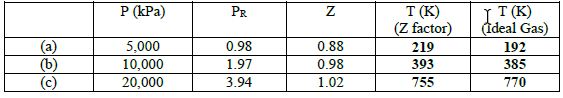
You might also like to view...
Heating cable for snow-melting is typically installed in a serpentine pattern with loops spaced at _____.
a. 6 inches (15 cm) on center b. 1 foot (30 cm) on center c. 1.5 feet (46 cm) on center d. 2 feet (61 cm) on center
The ____________________ Proficiency award is an FFA competition for FFA members who have job experience with turf, bedding plants, shrubs, and/or trees for production operations
Fill in the blank(s) with correct word
A garage 40' long and 28' wide is to be constructed using 8" block. The concrete footings are to be 2' wide and 8" thick. What is the length of the footing in feet?
What will be an ideal response?
_____ usually will not affect all the brakes at the same time, and the rig can be stopped.
A. Air blockage B. Brake fade C. Loss of air pressure D. Mechanical failure