This problem concerns a compound that has the following physical properties:
Critical Temperature = 1000 K
Critical Pressure = 25 atm
Boiling temperature at atmospheric pressure = 400 K
Molar Volume of saturated liquid at atmospheric pressure = 0.3 L/mol
Enthalpy of vaporization at atmospheric pressure = 15 kJ/mol
In the gaseous or vapor phase, is described by the equation of state
PV = RT + (BP3)
Where B = 0.05 L-mol-1atm-2and R = 0.08206 L-atm/mol-K = 8.314 J/mol-K
A) Derive an algebraic (i.e., no differentials or integrals) expression for the fugacity of this compound as a function of temperature, pressure, and known constants.
B) Give your best estimate of the fugacity of this compound in the vapor phase at T=500 K and P=10 atm.
C) Give your best estimate of the fugacity of this compound in the liquid phase at T=500 K and P=10 atm.
D) Based on your answers to B and C, is this compound a liquid or a vapor at T=500 K and P=10 atm?
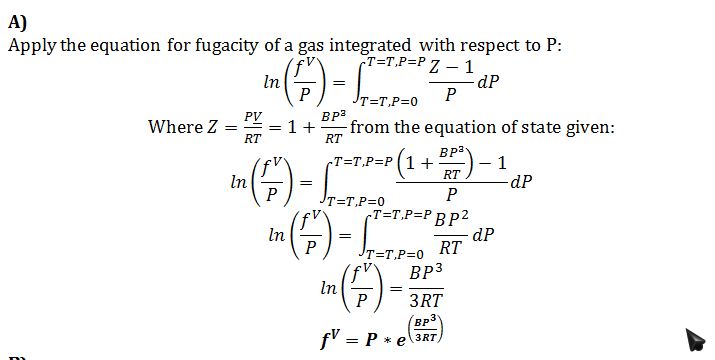
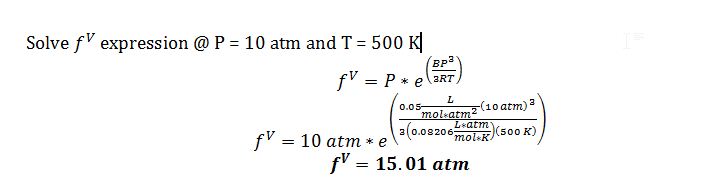
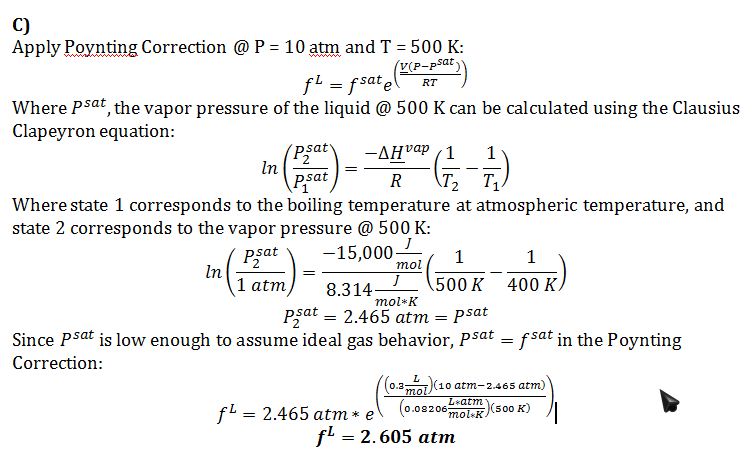
The compound is a liquid @ P = 10 atm and T = 500 K, which is shown both by f^L
You might also like to view...
Technician A says installing a thrust washer between the oil pump and the direct or front clutch will correct torque converter end play. Technician B says the torque converter must be installed in the transmission before checking end play. Who is correct?
A. A only B. B only C. Both A and B D. Neither A nor B
What is the most significant factor in the growth of the global transportation system?
What will be an ideal response?
What impact do stressors have in the systems theory?
What will be an ideal response?
The correct term for a coil using a low side float is ____________________.
Fill in the blank(s) with the appropriate word(s).