In Prob. 16.7 the volume fraction of the precipitate was taken to be 0.001.
(a) Assuming that the precipitate consists of cementite, and the the densities of cementite and ferrite are nearly equal, estimate the carbon concentration in the iron.
(b) Would it be possible to get this much carbon in solution at 1000 K? In other words, could one reasonably expect to obtain a 0.001 volume fraction of cementite precipitate on aging just above 300 K after a rapid quench from 1000 K?
Solution:
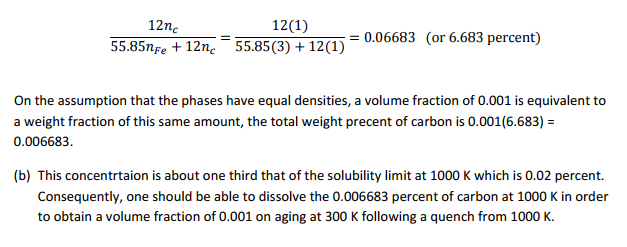
(a) What one needs to know is whether a volume fraction of cementite precipitate, equal to 0.001, contains less carnon than the solubility limit of carbon in the ? phase at 1000 K, which is 0.02 weight percent. At room temperature the solubility of carbon in iron is extremely small and for practical purposes may be assumed to be zero. Consequently, the equilibrium whose carbon concentration can be determined as follows. At this point it should be noted that cementite is Fe3C so that the ratio of iron atoms to carbon is 1 to 3. In additon, the atomic weight of iron is 55.85 and carbon 12. Thus the weight fraction of carbon in cementite is:
You might also like to view...
The four sides of a picture frame consist of two pieces selected from a population whose mean length is 30 cm with standard deviation 0.1 cm, and two pieces selected from a population whose mean length is 45 cm with standard deviation 0.3 cm.
a. Find the mean perimeter. b. Assuming the four pieces are chosen independently, find the standard deviation of the perimeter.
The estimated revenue generated by the environmental services industry in the United States is in excess of _____
a. $100 million per day b. $100 billion per year c. $100 billion per month d. $100 million per year
Before checking a system's operating superheat, the technician should first check:
A) The compressor contactor. B) The evaporator airflow. C) The condenser subcooling. D) The outdoor wet bulb temperature.
A parallel heat reclaim system primarily recovers:
A) Latent heat from the refrigerant. B) Sensible heat from the refrigerant. C) Both sensible and latent heat from the refrigerant.