It is possible for a mixture of hot water (90 °C) and ice to have a final equilibrium temperature of 0 °C
What will be an ideal response?
Absolutely. It depends on the masses of ice and water. The energy lost by the hot
water is m
hot water x [4186 J/(kg °C)] x (90 °C) = mhot water x 377 kJ. The energy gained
by the ice is mice x 333 kJ/kg. As long as mhot water x 377 kJ < mice x 333 kJ/kg the
system will remain at 0 °C.
You might also like to view...
RLC Circuits: The figure shows a series ac circuit. The inductor has a reactance of 60 ? and an inductance of 150 mH. A 50-? R and a capacitor C whose reactance is 90 ? are also in the circuit, and the rms current in the circuit is 1.5 A. What is the rms voltage of the source? 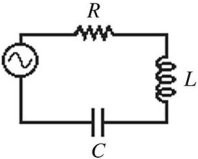
A. 87 V B. 84 V C. 81 V D. 78 V E. 75 V
The great contribution of Tycho Brahe was to
A) observe planetary positions with sufficient accuracy that later enabled Kepler to discover the laws of planetary motion. B) discover four moons orbiting Jupiter, thereby lending strong support to the idea that the Earth is not the center of the universe. C) offer the first detailed model of a Sun-centered solar system, thereby beginning the process of overturning the Earth-centered model of the Greeks. D) discover that planets orbit the Sun in elliptical orbits with varying speed.
A 10.0-? resistor, 10.0-mH inductor, and 10.0-?F capacitor are connected in series with a 10.0-kHz voltage source. The rms current through the circuit is 0.200 A. Find the rms voltage drop across each of the 3 elements.
What will be an ideal response?
When stars are moving away from us the color of their light is shifted toward the ______ (red or blue) end of the light spectrum because the wavelegth is ________ (shorter or longer).
a. red; longer b. red; shorter c. blue; longer d. blue; shorter