Five kilograms of liquid carbon tetrachloride undergo a mechanically reversible, isobaric change of state at 1 bar during which the temperature changes from 0°C to 20°C. Determine 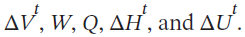 The properties for liquid carbon tetrachloride at 1 bar and 0°C may be assumed independent of temperature: ? =
The properties for liquid carbon tetrachloride at 1 bar and 0°C may be assumed independent of temperature: ? = 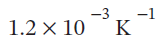
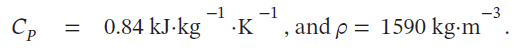
What will be an ideal response?
For an isobaric process with a substance with constant coefficient of thermal expansion, the change in volume is given by
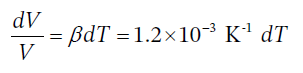
Integrating this over finite temperature gives
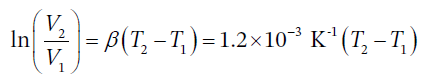
So, when the temperature increases by 20 degrees, the specific volume change is given by
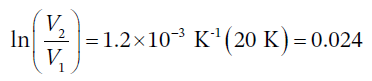
The initial specific volume is 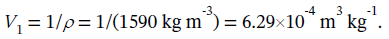
The final specific volume is then
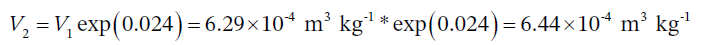
So, the change in specific volume is 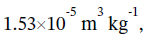 and the change in total volume is
and the change in total volume is 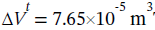 The work done is simply
The work done is simply 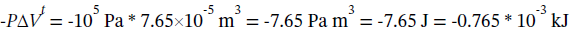 (the carbon tetrachloride does 7.65 J of work on its surroundings when it expands by
(the carbon tetrachloride does 7.65 J of work on its surroundings when it expands by 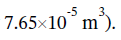
The heat required is 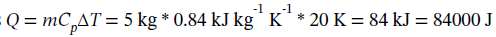 (more than 1000 times greater than the work done).
(more than 1000 times greater than the work done).
The enthalpy change for the constant pressure process is equal to 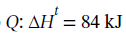
The internal energy change is 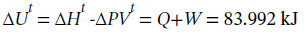
As is generally the case for liquids, which are nearly incompressible, the internal energy change and the enthalpy change are almost identical.
You might also like to view...
Carcass analysis is used to find out if there are changes in the amounts of specific tissues such as individual muscles
Indicate whether the statement is true or false
Which type of refrigerant hose is used with an R-134a system?
A) Barrier hose B) Rigid hose C) Copper type hose D) Flexible, porous hose
Campers being heated by a fire outside is an example of:
A) Sublimation. B) Radiation. C) Conduction. D) Convection.
One disadvantage of infrared thermometers is that they:
A) Are generally less accurate than dial-type pocket thermometers. B) Can only read the temperature of objects. C) Are only available in limited quantities due to their specialized nature. D) Take longer to get a reading than other types of thermometers.