Calculate the heat capacity of a gas sample from the following information: The sample comes to equilibrium in a flask at  and 121.3 kPa. A stopcock is opened briefly, allowing the pressure to drop to 101.3 kPa. With the stopcock closed, the flask warms, returning to
and 121.3 kPa. A stopcock is opened briefly, allowing the pressure to drop to 101.3 kPa. With the stopcock closed, the flask warms, returning to  and the pressure is measured as 104.0 kPa. Determine
and the pressure is measured as 104.0 kPa. Determine 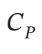 in
in 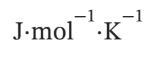 assuming the gas to be ideal and the expansion of the gas remaining in the flask to be reversible and adiabatic.
assuming the gas to be ideal and the expansion of the gas remaining in the flask to be reversible and adiabatic.
What will be an ideal response?
Let step 12 represent the initial reversible adiabatic expansion, and step 23, the final constant-volume heating.
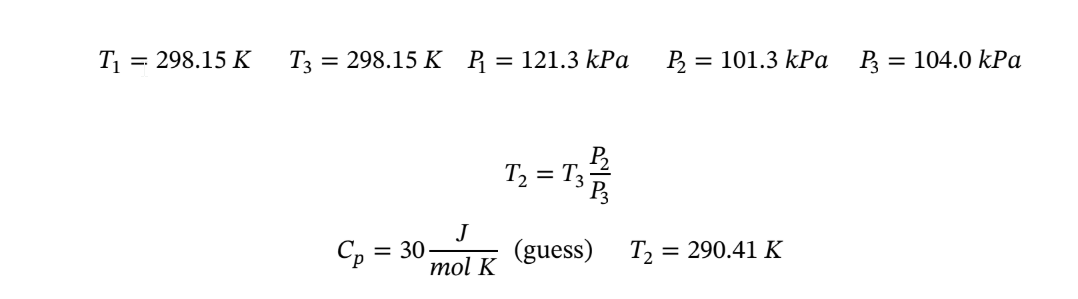
Given
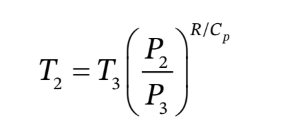
Solve for Cp
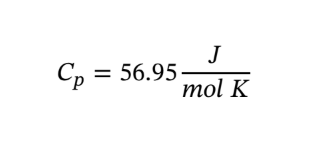
Trades & Technology
You might also like to view...
Many natural resources will not last forever
Indicate whether the statement is true or false
Trades & Technology
Which of the following is a perfect protein to meet human nutritional needs?
a. beef c. chicken eggs b. fish d. soybeans
Trades & Technology
In the graph, identify the coordinates of the point represented by the letter C.
Fill in the blank(s) with the appropriate word(s).
Trades & Technology
What is the zero point of an instrument whose 0% output is 5 PSIG and whose 100% output is 15 PSIG?
A) 15 psig B) 5 psig C) 10 psig D) 0 psig
Trades & Technology