100 grams of liquid nitrogen at 77 K is stirred into a beaker containing 200 grams of 5°C water. If the nitrogen leaves the solution as soon as it turns to gas, how much water freezes? (The heat of evaporation of nitrogen is 6.09 cal/gram and the heat of fusion of water is 80 cal/gram.)
none
You might also like to view...
Suppose that, for some unknown reason, the core of the Sun suddenly became hotter and the rate of nuclear fusion thereby increased. What would happen next?
A) The Sun would quickly run out of hydrogen, causing the temperature to return to its original value. B) The rate of fusion would almost instantly skyrocket, causing the Sun to explode. C) The temperature would continue to increase, causing higher and higher fusion rates. D) The core would expand, reducing the pressure and temperature, and the rate of fusion would decrease until it returned to its original level.
A 6.0-kg object moving 2.0 m/s in the positive x direction has a one-dimensional elastic collision with a 4.0-kg object moving 3.0 m/s in the opposite direction. What is the total kinetic energy of the two-mass system after the collision?
a. 30 J b. 62 J c. 20 J d. 44 J e. 24 J
The two masses in the figure are released from rest. After the 3.0-kg mass has fallen 1.5 m, it is moving with a speed of 3.8 m/s. What is the change in mechanical energy done on the system during this time interval by the frictional force on the 2.0 kg mass?
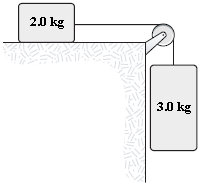
a.
?12 J
b.
?17 J
c.
?20 J
d.
?8.0 J
e.
?28 J
If the average translational kinetic energy of the molecules in one sample of gas has twice the value of that of another sample of gas and the temperature of the first sample of gas is 100°C, what is the temperature of the second sample of gas?
1.100°C 2.50°C 3.0°C 4.-86.6°C