If 33 atom % Cu is mixed into a Ni crystal at a temperature of 473 K (200°C), determine the change in the GFE relative to segregated Cu and Ni for 1 mole of total atoms if the enthalpy of solution for Cu into Ni is 0.11 eV/atom, according to data in Hultgren et al.
(a) What is the GFE change for the mixing?
(b) Based upon your result, should it be possible to mix 33 atom % Cu into Ni at 473 K? Explain your answer and compare this result to the phase diagram in Figure 5.7.
(a) To determine the entropy of mixing the 33% copper into nickel for a total of one mole of atoms, the chemical composition values are inserted into the mixing entropy equation. Since the problem is on a per mole basis R replaces k in the mixing entropy equation.

You might also like to view...
If the distance to the nearest star is 4.2 light-years, then
a. the star is 4.2 million AU away. b. the light we see left the star 4.2 years ago. c. the star must have formed 4.2 billion years ago. d. the star must be very young. e. the star must be very old.
Equilibrium: An athlete holds a 7.5-kg shot put in his hand with his lower arm horizontal, as shown in the figure. His lower arm has a mass of 2.8 kg and its center of gravity (or center of mass) is 12 cm from the elbow-joint pivot. How much force must the extensor muscle (which is M in the figure) in the upper arm exert on the lower arm?
M in the figure) in the upper arm exert on the lower arm?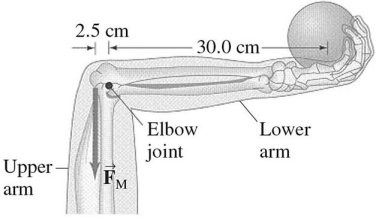
A. 100 N B. 500 N C. 1000 N D. 1500 N
The expression(s) indicating the mass number of a nucleus is
a. A. b. N. c. Z. d. Z + N. e. A – Z.
When water freezes, it expands about nine percent. What would be the pressure increase inside your automobile engine block if the water in there froze? The bulk modulus of ice is 2.0 × 10^9 N/m2, and 1 atm= 1.01 × 10^5 N/m2
a. 18 atm b. 360 atm c. 1100 atm d. 1600 atm e. 600 atm