If the final temperature was determined incorrectly (a value greater than the true value was obtained), how would this affect the calculated value of the specific heat? Explain your answer
The value for the specific heat will be much greater because the temperature
difference for the water and the calorimeter cup will increase and the temperature
difference for the metal will decrease. The specific heat for the metal is determined
by dividing water and cup values by the metal values.
You might also like to view...
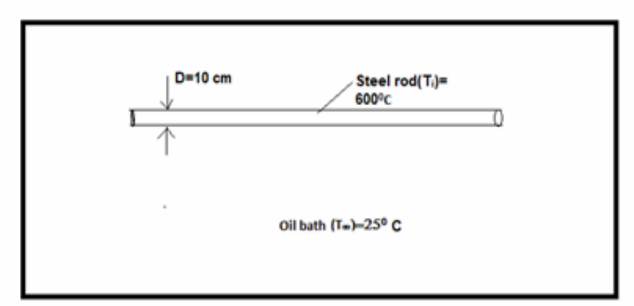
Consider a heat treatment process in which steel rods with a 10 cm diameter at an initial temperature of 600°C are inserted into an oil bath at 25°C. Assuming a convection coefficient of 400 W/(m2 K) between the oil and the rod, estimate how long it takes for the centerline of the rod to cool to 60°C. If the steel rod, which is 1 m long, is withdrawn from the bath at this point in the process, i.e., when the centerline temperature has reached 60°C, estimate the rate at which heat must be extracted from the oil to maintain its temperature constant during the process of treating 25 rods per hour.
GIVEN
• Heat treatment of steel rod inserted in oil bath
• Rod outside diameter = 0.1 m
• Initial temperature (T0) = 600°C
• Oil bath temperature (T?) = 25°C
• The average heat transfer coefficient ch = 400 W/(m2 K)
FIND
• Time it takes for centerline of the rod to cool to 600C.
• Rate at which heat must be extracted from oil to maintain its temperature constant during the process of treating 25 rods per hour.
ASSUMPTIONS
• Radial conduction only in billet
• Uniform and constant properties
SKETCH
Consider an infinite pipe that conducts current along its length. The magnetic field that this pipe creates is
A. unidirectional. B. circular. C. axial. D. None of the above.
An FM radio station broadcasts at 98.6 MHz. What is the wavelength of the radiowaves?
a. 60.8 m b. 6.08 m c. 3.04 m d. 0.314 m e. 0.33 cm
Why might increasing the concentration of a set of reactants increase the rate of reaction?
A) You have increased the chances that any two reactant molecules will collide and react. B) You have increased the ratio of reactants to products. C) The concentration of reactants is unrelated to the rate of reaction. D) The rate of reaction depends only on the mass of the atoms and therefore increases as you increase the mass of the reactants. E) none of the above